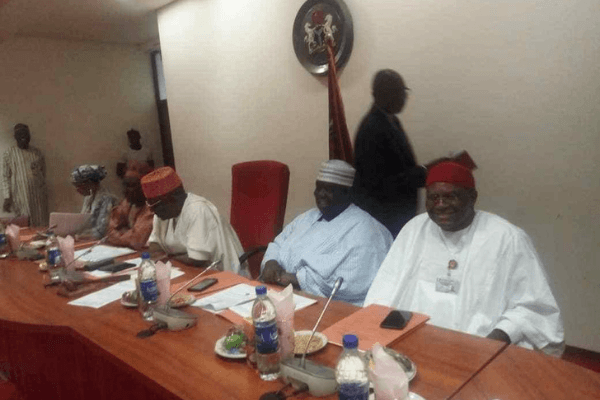
National Agency for Food and Drug Administration and Control (NAFDAC) has exonerated the National Eye Centre, in the Avastin crisis where 10 patients were said to have lost their eyesight.
The agency in its laboratory test report says the Avastin drug conforms with standard certification process, as the drug’s storage and chemical composition are excellent.
Read Also : Here are the reasons why your child’s eyes needs early attention
Speaking on behalf of the director-general of NAFDAC before the Senate ad-hoc committee Wednesday, the director of Pharmacovigilance, Ali Ibrahim, maintained that, “before we registered the avastine drug for use in Nigeria, it was tested and proven to be effective, apart from the monitoring and effective tracking.”
NAFDAC however said the National Eye Centre patient had some side effects already indicated on the drug usage. The Senate ad-hoc committee while commending the National Eye Centre, said, “Since the hospital has used the drug on 2113 patients in the past it could be that the patients actually had inflammation as stated as the drug’s side effects.”
The Senate ad-hoc committee chairman of the ad-hoc committee, Matthew Urhoghide, representing Edo South Senatorial District, also stressed that doctors in National Eye Centre must always exercise caution in administering all drugs to avoid challenges, and must not be discouraged because of what happened but continue to restore sight to Nigerians.
Also speaking, the Chief Medical Director of the Eye Centre, Mahmoud Alhassan, reiterated the Centre’s commitment towards restoring sight of the avastin patients, stressing that N7.6 million had been spent on treatment, feeding and general welfare of the affected patients.